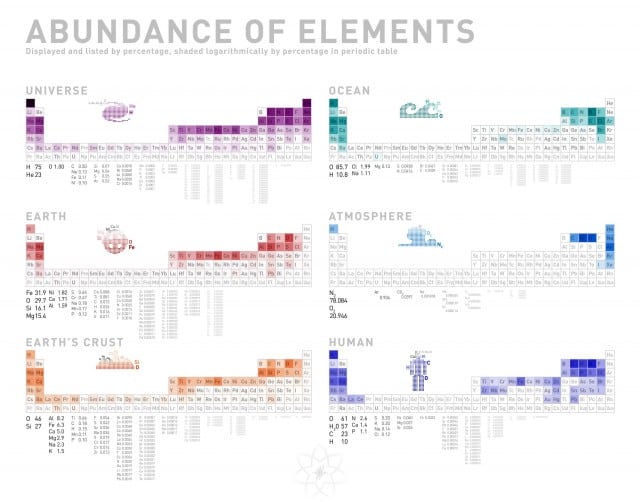
It truly boggles the mind just how many factors go into making life possible here on Earth, especially when you think of everything as being composed of bajillions of different atoms mashed together. Everything in the universe breaks down into chemistry, and this infographic attempts to visualize that fact.
Chemistry is the science of stuff (i.e. the things we’ve put on the periodic table) and stuff = atoms. When you have matter of some sort, it contains atoms comprised of many different combinations of protons, neutrons, and electrons. These different combinations define the elements. For instance, if there are 79 protons in an atom, then that atom is the element gold and will continue to be gold as long as there are 79 protons in that atom. The number of protons is also that element’s atomic number (this is because it is unlikely for the proton number to change in an atom). Electrons on the other hand can be variable and can change their number easily and often.
Now, this infographic shows the approximate percentage of every element in the universe, ocean, earth, crust, atmosphere and inside us. I thought it was interesting that most of the lower numbers (atomically speaking) are more prevalent throughout all of these areas than the higher atomic numbered elements. I suppose this makes sense as stars typically produce simple atoms first and thus would be much more common in the universe than say, Ununpentium. SCIENCE!
[via]